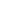U.S. vaccination plods on as COVID-19 claims over 700,000 lives
Merck and Ridgeback Biotherapeutics said they created an antiviral pill that can reduce the risk of COVID-19 hospitalization and death by about 50 percent, but health experts cautioned that it wasn't a replacement for vaccinations, which remain the most effective path to ending the coronavirus pandemic if enough people get their shots.
NEW YORK, Oct. 2 (Xinhua) -- The United States has set the pace to expand the spectrum of COVID-19 vaccines, while its vaccination campaign drags on as it hit a grim milestone of 700,000 deaths in the pandemic that has entered its 19th month.
Topping the world, the United States on Friday surpassed 700,000 deaths from COVID-19, according to Johns Hopkins University's data. Meanwhile, the average number of people getting vaccinated, at 270,531 per day, is the lowest it has been since Aug. 15, according to the U.S. Centers for Disease Control and Prevention (CDC).
The CDC updated on Saturday that 214,597,690 people have received at least one dose of COVID-19 vaccine, making up 64.6 percent of the whole U.S. population; fully vaccinated people stood at 184,852,416, accounting for 55.7 percent of the total. A total of 4,363,791 people, or 2.4 percent of the fully vaccinated group, received booster shots.
Trying to add some bright color to the ongoing tragedy, CDC Director Rochelle Walensky told reporters at a White House briefing on Friday that weekly COVID-19 cases and hospitalizations in the United States were down by 15 percent from the previous week.

A man sits beside white flags placed on the National Mall to honor the lives lost to COVID-19 in Washington, D.C., the United States, Sept. 18, 2021. (Photo by Aaron Schwartz/Xinhua)
According to The New York Times' update, the seven-day average of confirmed cases of the pandemic stood at 109,192 nationwide on Friday, with its 14-day change striking a 27-percent fall. The COVID-19-related deaths were 1,883 on Friday, with the 14-day change realizing a 5-percent decrease.
WIDE-RANGING VACCINE REQUIREMENTS
"As many companies impose COVID-19 vaccine mandates, employees who refuse to get jabbed are getting the ax," reported CBS on Friday, adding that employers in the health care and aviation industries this week dismissed hundreds of workers who declined to get inoculated.
A case in point is Christiana Care, which is headquartered in Wilmington, Delaware, and has 1,200 beds across three hospitals. On Monday, it announced that some 150 of its employees had failed to meet a Sept. 21 deadline to be fully vaccinated. As a result, they were fired.
Health care organizations account for most of the firings of unvaccinated workers, but other kinds of employers are also expected to follow suit when workers start reporting to offices again in earnest.
Currently, a dilemma is being weighed for some companies considering vaccine mandates: an acute worker shortage among retailers, restaurants, hotels and other service-sector employers, according to the report.

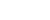
Pedestrians walk past a broadway theater in New York, the United States, July 2, 2021. (Xinhua/Wang Ying)
On Friday, the Broadway League announced that all its 41 theaters in New York City will continue to require COVID-19 vaccinations for audience members, performers, backstage crew and venue staff at least through the end of the year.
Audiences also will be required to wear masks inside the theaters at least for the remainder of 2021, except while actively eating or drinking in designated locations.
The announcement extended the COVID-19 protocol policy that has been in place since Broadway resumed performances in July. At that time, the League said it would review the procedures in the fall. Theater owners will again review the policies by Dec. 1 for performances starting Jan. 3, 2022.
"We know that with these policies in place through the end of the year, we will continue to help our audiences feel safe and to deliver them the thrill of Broadway night after night," Charlotte St. Martin, president of the Broadway League, said in a statement.
PILLS, MORE VACCINES
Merck and Ridgeback Biotherapeutics said on Friday that they created an antiviral pill that can reduce the risk of COVID-19 hospitalization and death by about 50 percent, but health experts cautioned that it wasn't a replacement for vaccinations, which remain the most effective path to ending the coronavirus pandemic if enough people get their shots.
"This can be used in conjunction with the vaccine. And it's not an alternative to vaccination. We still have to try to get more people vaccinated," Scott Gottlieb, former commissioner of the U.S. Food and Drug Administration (FDA), was quoted by CNN as saying. Gottlieb acknowledged that the antiviral medicine could be effective for those who choose not to get vaccinated as well as those who catch the virus while fully vaccinated.
Merck said that it will seek FDA emergency use authorization for its molnupiravir medication "as soon as possible." If permitted, it would become the first oral medicine that fights viral infection for COVID-19.
"If approved, I think the right way to think about this is this is a potential additional tool in our toolbox to protect people from the worst outcomes of COVID," White House COVID-19 Response Coordinator Jeff Zients said on Friday.

Photo taken on Aug. 23, 2021 shows the U.S. Food and Drug Administration in Silver Spring, Maryland, the United States. (Photo by Ting Shen/Xinhua)
Also on Friday, the FDA announced that its independent vaccine advisory committee will hold three meetings in October to discuss COVID-19 booster shots, mix-and-match boosters and vaccines for children aged 5 to 11.
The first two meetings, on Oct. 14 and 15, will cover booster doses of the Moderna vaccine and the Johnson & Johnson vaccine, both of which are authorized for use in adults. During the second meeting, the committee also will discuss data from the National Institutes of Health and the safety and efficacy of getting initial doses of one COVID-19 vaccine and, later, a booster dose of another manufacturer's shot.
Less than two weeks later, on Oct. 26, the FDA panel will discuss Pfizer's request to FDA to authorize its COVID-19 shot for 5 to 11-year-old children. No vaccines are currently available for kids under 12, and only Pfizer's shot is available for teens 12 to 17.
"The meetings set up a rough timeline for a slate of FDA decisions that could help the country avoid a damaging winter surge, and ultimately help bring the pandemic to an end," reported U.S. news portal Politico.
Photos
Related Stories
- More than half of U.S. citizens distrust gov't on COVID-19 issues: poll
- Polarization between parties fuels COVID-19 vaccine reluctance in U.S.: The Atlantic
- Fitch warns debt limit brinkmanship could put pressure on U.S. triple-A rating
- Clock ticks toward Friday as U.S. gov't shutdown looms
- Pumpkins seen at Arboretum's Pumpkin Village in Texas, U.S.
Copyright © 2021 People's Daily Online. All Rights Reserved.