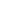U.S. starts clinical trial of mRNA universal influenza vaccine

A medical worker administers a dose of flu vaccine to a recipient at a medical center in Rosemead, California, the United States, on Dec. 10, 2022. (Xinhua)
This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP, for safety and its ability to induce an immune response, according to the NIH.
LOS ANGELES, May 15 (Xinhua) -- The United States has started a clinical trial of an experimental universal influenza vaccine developed by researchers at the U.S. National Institutes of Health (NIH), the agency said on Monday.
The trial has begun enrolling volunteers at Duke University in Durham, North Carolina. This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP, for safety and its ability to induce an immune response, according to the NIH.
The trial will enroll up to 50 healthy volunteers aged 18 through 49. Three groups of study participants, 10 participants each, will be vaccinated with 10, 25 and 50 micrograms of the experimental vaccine, respectively.
After evaluation of the data to determine an optimum dosage, an additional 10 participants will be enrolled to receive the optimum dosage.
The study also will include a group of participants who will receive a current quadrivalent seasonal influenza vaccine. This will allow the researchers a point of direct comparison between the immunogenicity and safety of the candidate vaccine and available seasonal flu vaccines, according to the NIH.
Photos
Related Stories
- China issues new COVID-19 vaccination plan to further curb risks
- Chinese scientists develop new vaccine strategy by entrapping live virus into hydrogel
- Unequal access to COVID-19 vaccines causes preventable deaths: open letter
- Clinical trial finds temperature-stable TB vaccine safe, prompts immune response
- New study shows COVID-19 vaccination linked to fewer cardiac events
Copyright © 2023 People's Daily Online. All Rights Reserved.